State Laws
Allergy & Asthma Network has joined with patient advocates to lead numerous efforts to champion federal and state laws to improve the health and quality of life for people with asthma, allergies and related conditions. Following are key pieces of legislation at the state and federal levels that are important for people with these conditions to learn about and be aware of.
Student rights to self-carry epinephrine for anaphylaxis
Student rights to self-carry albuterol inhalers for asthma
Stocking schools with epinephrine for treatment of anaphylaxis
Stocking schools with albuterol for treatment of asthma
Entity/public place stock epinephrine
Step therapy
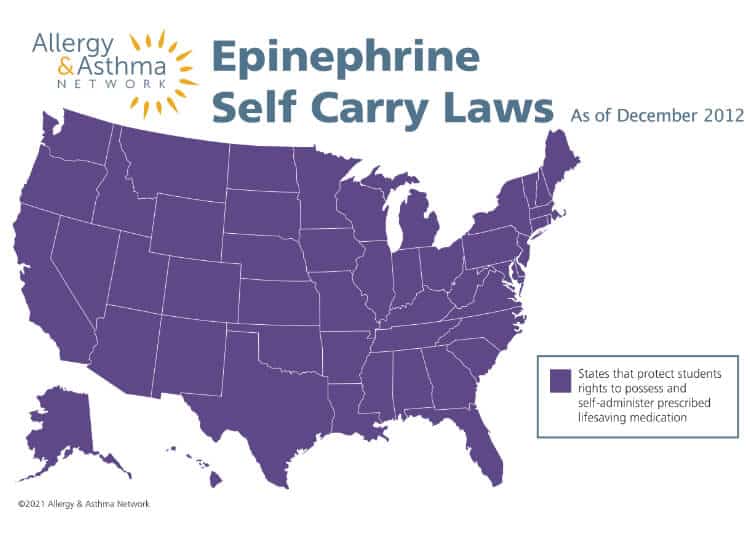
Epinephrine Self Carry Laws
Epinephrine self-carry laws across the country authorize students with severe allergies to possess and self-administer prescribed epinephrine, the first line of defense to treat an anaphylaxis emergency.
Why is this important?
Student have rights to self-carry epinephrine auto-injectors.
All 50 states now have laws protecting students’ rights to carry and use prescribed anaphylaxis medications.
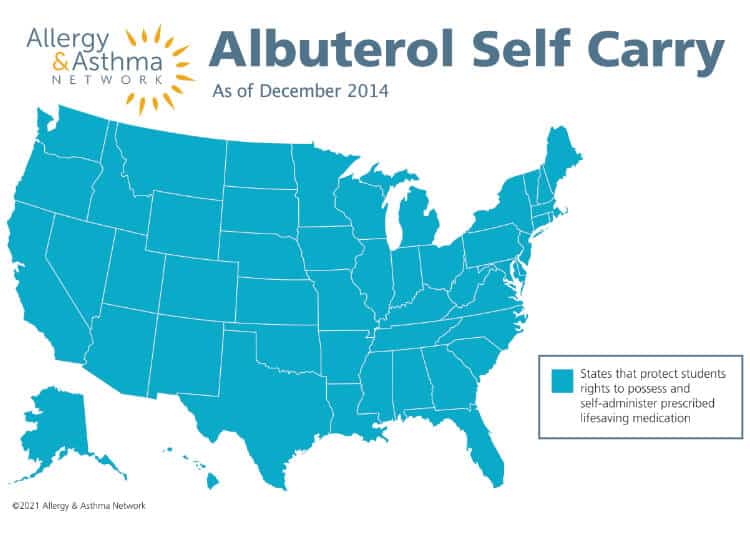
Albuterol Self Carry Laws
Albuterol self-carry laws across the country authorize students with chronic respiratory conditions (i.e., asthma) to possess and self-administer prescribed albuterol mediation
Why is this important?
Student have rights to self-carry albuterol inhalers.
All 50 states now have laws protecting students’ rights to carry and use prescribed asthma medications.

School Stock Epinephrine Laws
Most states now have laws that allow or require anaphylaxis emergency preparedness plans that permit schools to stock emergency supplies of epinephrine auto-injectors.
Why is it important for schools to stock epinephrine?
Stock epinephrine laws across the country help save the lives of students who experience anaphylaxis at school and do not have a prescribed epinephrine auto-injector. Access at school is critical because 25 percent of anaphylaxis reactions at school occur in students previously undiagnosed with a severe allergy to food, insect venom, latex or medication.
The stock epinephrine in schools law was often the first of its kind to deal with stocking non-patient specific emergency medications in the school setting. They have since been used as a template or foundation for other emergency medications.
What can I do if my child’s school does not stock epinephrine?
If your state has a law, there could be issues with implementing the law at your child’s school.
If there is not an existing state law, there is still work to be done in statehouses, legislatures and schools as lawmakers and policy makers develop law and implementation policies.
See Allergy & Asthma Network’s recent article with video about stock epineprhine and stock albuterol.
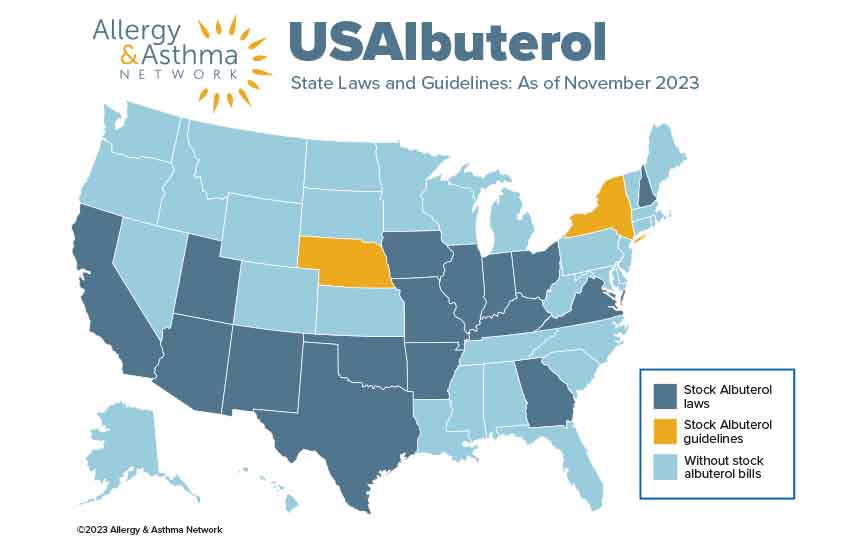
School Stock Albuterol Laws
A number of states across the country have passed laws or guidelines that permit schools to stock albuterol with a prescription and administer it to a student believed to be in respiratory distress and standardize asthma action plans.
This type of preparation and management in schools will not only improve a child’s health, but will also ensure students are able to focus on learning.
Currently 18 states (e.g., Arizona, Arkansas, California, Georgia, Illinois, Indiana, Iowa, Kentucky, Missouri, Nebraska, New Hampshire, New Mexico, New York, Ohio, Oklahoma, Texas, Utah and Virginia) have laws or guidelines that permit schools to stock albuterol inhalers with a prescription and administer it to a student believed to be in respiratory distress.
At the federal level, the “School-Based Allergies and Asthma Management Program Act” became law in January 2021 to encourage more schools around the country to have proper training and implement comprehensive school-based asthma and allergy management programs (SAMPRO) by increasing federal grant preferences to states.
Check the status of your state in the map below. States colored light blue do not have stock albuterol legislation.
Why is it important for schools to stock albuterol?
Stock albuterol laws across the country help save the lives of students who experience an asthma attack at school and do not have a prescribed albuterol inhaler with them.
See Allergy & Asthma Network’s recent article with video about stock epineprhine and stock albuterol.
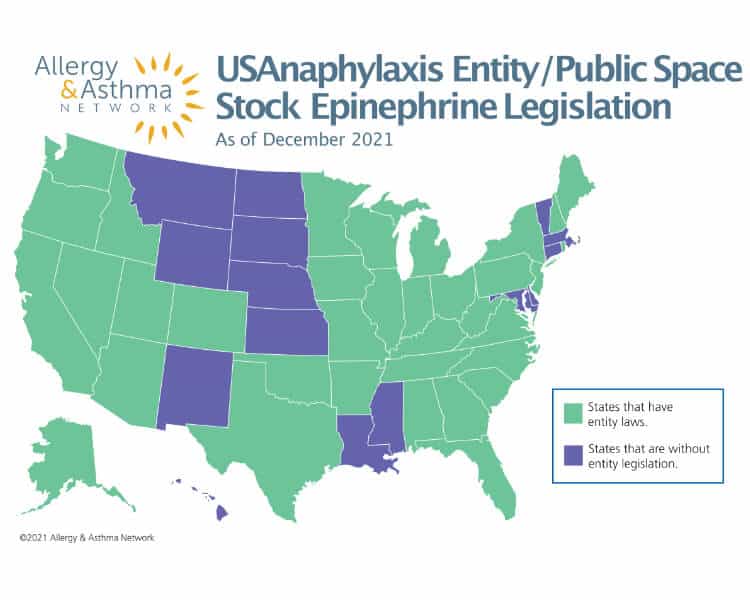
Entity/Public Place Stock Epinephrine Laws
Stock epinephrine has now moved beyond the school walls and out to public venues – from theme parks, restaurants and sports arenas to daycare centers and more. Many states passed entity epinephrine laws that permit public venues to stock emergency supplies of epinephrine auto-injectors.
Why is it important for entities and public places to stock epinephrine?
These laws allow restaurants, theme parks and other public entities to respond to a life-threatening allergic reaction in the event someone experiences anaphylaxis and does not have an epinephrine injector with them.
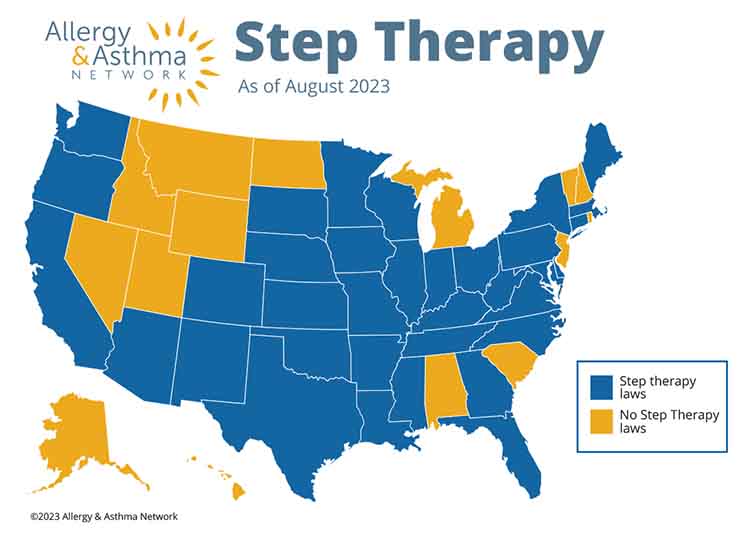
Step Therapy Laws
Step therapy is a practice health insurance plans use to manage the cost of medications. Laws have been passed in a number of states to help provide a clear and transparent process to seek exceptions and approvals for medications subject to step therapy, and to establish a reasonable and clear timeframe for overriding decisions made by the healthcare provider.
Please contact us if you need information about existing legislation in your state.
For more information on additional state issues on which we engage, or to find out how you can get involved, please view our state advocacy agenda and visit our “Advocacy Action Center”.
